Learning, Memory, & Social Development - Connections to Nutrition
Throughout life, and especially in children, opportunities to interact, learn, and create meaningful memories arise. How can these opportunities be supported through nutrition and its influence on brain development?
Here, we explore the impact of a group of key nutrients, including on the white (myelin) and gray brain matter development to lay the foundation for the learning process, as well as the maturation of sleep patterns which are linked to memory consolidation, and finally, social-emotional development.
Let’s start by breaking down the learning process into these steps:
1) Information is received by our body (interactions, hearing, seeing, etc.).
2) Information received is made sense of (brain processing).
3) This knowledge is then stored as memories.
Think about what happens when a child picks up a toy car for the first time.
- The moment he sees and touches it, he knows how it looks and feels.
- He thinks about what it does and learns what he can do with it as he plays with it.
- Finally, he remembers what the toy is and does.
This is learning at its simplest yet occurs through such a sophisticated process in the brain.
This transport of information across different part of the brain responsible for motor, visual and auditory functions, etc., needs to happen at lightning speed.
In fact, literal electricity passes through the brain as electrical currents and must be done accurately and precisely, arriving where they need to be at, almost akin to a sophisticated train system. Modern mapping methods such as MRI imaging have been developed to understand this brain’s ‘subway map’.
Constructing the brain ‘subway map’ through nutrition
The constructing of this brain ‘subway map’ happens rapidly during the first 24 months from birth, with myelination being an important building step. Myelination builds an insulative coating (the myelin sheath) around the connecting ‘railways’ between the brain cells, increasing electrical speed up to 100-times faster. Targeted nutrition provides the building blocks to make this sheath.
Connecting the science behind brain-building nutrients
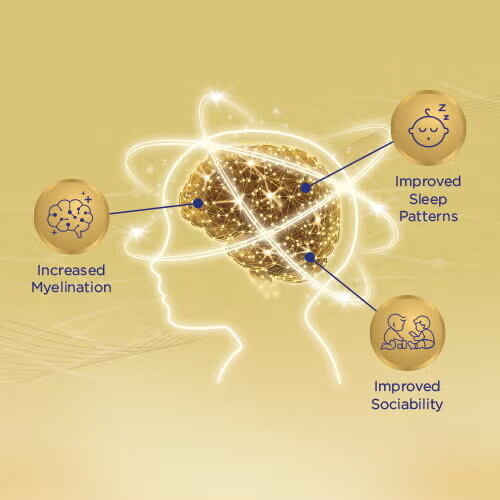
As expected, breastfed children show the highest myelination and intelligence measurements. But until recently, the way brain-building nutrients work together to support this was not explored. Thus in 2020, the first-ever clinical study to observe the effects of a formula containing a blend of myelin-promoting nutrients was carried out. Results from the 24-month MRI imaging and brain assessments showed increased myelin in the brain ranging up to 32% more, especially in areas typically associated with skills needed for learning such as reading, language processing and working memory.
Furthermore, increased day sleep and fewer night awakenings were found, which may support optimized memory building at night. Going back to example of playing with a toy, as the child gathers new information during his interactions, he needs time to rest, process and remember what he has learnt from the session.
Reduced social fearfulness in new environments was also shown. Social fearfulness is part of normal social development and is linked to day-to-day interactions, such as meeting others for the first time. This is important as being able to interact with new friends is part of playing and learning in new environments.
Summary
Opportunities to explore and learn abound in childhood as the brain develops, with nutrition providing the building blocks. Myelination is key to this brain development, speeding up information transfer and processing to support learning. Using the latest brain imaging technology has for the first time demonstrated the positive effects of a combination of specific nutrients on brain myelination, building the foundations for learning, memory recall and social development.
References
Immordino-Yang, M. H., Darling-Hammond, L., & Krone, C. R. Educational Psychologist 2019;54(3):185-204.
McGann, J. P. Learning & Memory 2015;22(11):567-576.
Heckner, M. K., et al. Scientific Reports 2021;11(1):9942.
Persinger, M. A. Frontiers in Integrative Neuroscience 2012;6:19.
Telesford, Q. K., et al. Brain Connectivity 2011;1(4):295-308.
Ashworth, A., et al. Journal of Sleep Research 2014;23(3):304-310.
Rubinov, M., & Sporns, O. NeuroImage 2010;52(3):1059-1069.
Casey, B. J., et al. Trends in Cognitive Sciences 2005;9(3):104-110.
Deoni S. C. L. Nestle Nutrition Institute Workshop Series 2018;89:155–174.
Deoni, S. C. L. et al. NeuroImage 2018;178:649-659.
Simons, M., & Trotter, J. Current Opinion in Neurobiology 2007;17(5):533-540.
Monje, M. Annual Review of Neuroscience 2018;41:61-76.
Deoni S. C. L. Nestle Nutrition Institute Workshop Series 2018;89:155–174.
Schneider, N., et al. eNeuro 2019;6(4):1-13.
Schneider, N., et al. Frontiers in Nutrition 2022;9:823893.
Schneider, N., et al. Nutrients 2023;15(20):4439.
Nagy, Z., Westerberg, H., & Klingberg, T. Journal of Cognitive Neuroscience 2004;16(7):1227-1233.
O'Muircheartaigh, J., et al. Human Brain Mapping 2014;35(9):4475-4487. 7.
Scharf, R. J., Scharf, G. J., & Stroustrup, A. Pediatrics in Review 2016;37(1):25-38.